|
Tests at SPS Medical, an independent lab in Rush, N.Y., showed PPSU became
significantly yellowed and brittle after only 150 Sterrad NX cycles, while Ultem
HU1004 showed negligible yellowing or embrittlement after 300 cycles. PPSU
showed 10 times greater color shift than Ultem HU 1004 resin significantly
yellowing after only 100 Sterrad NX cycles.
With the Ultem HU1004 resin grade, Ultem resins are now one of the few materials
that can maintain its key performance properties after exposure to the four most
common techniques used in sterilization environments, including:
Finally, Abbott Vascular is working to develop a fully bioresorbable polylactic
acid (PLA) based stent. While there are three types of bioabsorbable stent
technologies under development - including bioabsorbable polymers to coat metal
stents, totally bioabsorbable metallic stents that corrode and totally
bioabsorbable polymer stents - many stent makers believe that fully dissolving
polymer stents are the long term solution. Metal stents are foreign objects that
can:
-
Irritate vessels and cause stent thrombosis
-
Prevent vessel remodeling
-
Prevent future surgical interventions
-
Can fatigue and suffer strut fractures, and impair CT or MR imaging
For these reasons, several stent manufacturers are working on biodegradable
stents that do not leave behind any hardware. The main challenge with a fully
dissolving polymer stent is to maintain radial strength to keep the vessel open
long enough to prevent renarrowing.
Abbott is using stretch blowmolding to enhance physical properties of PLA used
to develop its Absorb bioresorbable vascular stent. The stent needs to remain
intact for 3-6 months to support artery walls until they can stay open on their
own and dispense drugs that maintain artery health. The entire drug is fully
eluted in 120 days. The PLA is fully broken down within two years into water and
CO2.
PLA degradation rates can be adjusted by changing the ratio of crystalline
material in the PLA - the amorphous zones in the PLA breakdown at a different
rate than the semi-crystalline zones. Abbott is using strain induced
crystallization in the stretch blowmolding process to alter the crystalline
material ratio in the PLA. PLA strength is also tailored to provide the
necessary vascular wall support and to withstand the sterilization process.
Abbott commercialized the international launch of its Absorb bioresorbable
vascular scaffold, now widely available across Europe and parts of the Asia
Pacific and Latin America. Abbott is referring to Absorb as a scaffold to
indicate that it is a temporary structure, unlike a stent which is a permanent
implant.
The international launch of Absorb followed a medical trial program that
encompassed five studies in more than 20 countries. Abbott says that study data
indicate that Absorb performs similar to a best-in-class drug-eluting stent on
tests such as major adverse cardiovascular events (MACE) and target lesion
revascularization (TLR).
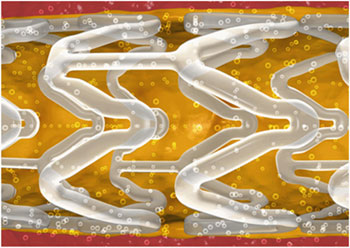
Abbott Vascular
Absorb bioresorbable vascular scaffold
http://exclusive.multibriefs.com/content/medical-device-plastic-material-innovations-to-watch
|
