|
Medical Device Innovator Spreads Winds In Country
Masimo, the inventor of breakthrough noninvasive patient
monitoring technologies, recently announced the opening of offices across India
to meet the health demands.
"We have found that India is an important market for several
reasons - the most important is that the penetration of high technology devices
is very low, a factor that could lead to thousands of avoidable instances of
death and illness. As a leader in noninvasive patient monitoring, including
measure through motion and low perfusion pulse oximetry, Masimo's technologies
will be able to make a significant impact to improve patient safety and
outcomes," said Jon Coleman, president, Masimo Worldwide Sales, Professional
Services and Medical Affairs.
Masimo is a publicly traded medical technology company. It is
working with India's medical and nursing practitioners to raise greater
awareness of the clinical and cost challenges that innovative technologies can
solve, including, pulse oximetry. Compared to other pulse oximeters during
patient motion and low perfusion, Masimo SET provides measurements which they
claim other pulse oximeters cannot. It dramatically reduces false alarms, and
accurately detects true alarms that can indicate a deteriorating patient.
http://timesofindia.indiatimes.com/business/india-business/Medical-device-innovator-spreads-winds-in-country/articleshow/29151064.cms
Medical Device Plastic Material Innovations To Watch
The medical device universe encompasses a particularly
imposing spectrum of constant technological innovation, including hundreds of
different technologies and thousands of types of products. This affords
high-performance specialty plastics material suppliers unique opportunities in
medical device market development.
High-purity medical plastics, and more specifically
high-purity medical thermoplastic vulcanizates (TPVs), are a good example.
There's a growing focus on extractables in healthcare supplies and equipment as
concerns grow about endocrine disruption and other health issues.
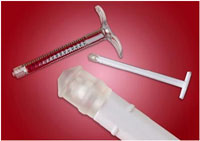
Teknor Apex Company
Medical plunger tip seals
Medalist MD-200 series TPVs from the Teknor Apex Company have
high purity and resilience suitable for replacing rubber in medical
applications. The materials are certified to pass the ISO 109993-5 materials
standard and have none of the extractable heavy metals commonly used in curing
thermoset rubber. They are also Drug Master File listed with the U.S. FDA.
These TPV medical elastomers span 15 to 80 Shore A hardness.
They also meet the requirements of ISO 7886 for single-use hypodermic syringe
stoppers and exhibit a low coefficient of friction (COF) for consistent travel
force in glass and PP barrels.
Next
|
