|
Stanford Brings Affordable Medical Innovation To India
Through Collaborative Design
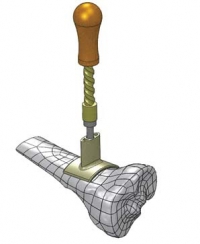 A BONE DRILL is a nasty-sounding device that looks like
something you'd find on the shelf at Home Depot. You wouldn't want to see one
coming at you, but if you needed one, you'd likely be in such bad shape that you
wouldn't even notice. A BONE DRILL is a nasty-sounding device that looks like
something you'd find on the shelf at Home Depot. You wouldn't want to see one
coming at you, but if you needed one, you'd likely be in such bad shape that you
wouldn't even notice.
The devices, which typically cost about $300 each, are used
to access the marrow and vascular system inside bones when a patient's veins
have collapsed or are inaccessible. They're standard features in most American
ambulances and emergency rooms.
But in developing countries like India, where the need is
huge, that $300 price is an insurmountable hurdle to widespread adoption. And
therein lies an opportunity. What if you could design a low-cost device to do
the same job, without the bells and whistles that make conventional ones
expensive?
That's exactly the kind of challenge that gets the fellows of
the Stanford India Biodesign (SIB) team excited. The program, a partnership with
the Indian government started in 2007, is based at Stanford and in New Delhi at
the Indian Institute of Technology and the All India Institute of Medical
Sciences. Each year, four Indian fellows interested in medical-technology design
and development go to Palo Alto for six months to learn the basics of the
Stanford-created biodesign process. They then head back to India, fanning out to
observe medical care in both high-volume urban hospitals and rural clinics.
Their mandate: Draw up a list of patient-care needs that might be solved with
better devices.
That model, where ideas for new products bubble up from
clinical practices observed in developing countries, is a relatively new one.
Typically, big companies would consult practicing doctors for suggestions, but
that only got them so far. "Physicians are generally good at identifying
incremental problems, suggesting, for example, a change in a handle," but not
good at imagining breakthrough technologies, says Dr. Rajiv Doshi, the U.S.
executive director of SIB and a consulting assistant professor in the Department
of Medicine at Stanford. Instead, revolutionary American innovations tend to
come from startups, which -- driven by market dynamics -- introduce products to
the developing world at high prices.
But in 2009, GE flipped that model on its head when it
introduced a $1,000 electrocardiogram device and a $15,000 portable ultrasound
machine that it had developed in rural India and in rural China, respectively.
That new paradigm, dubbed "reverse innovation," has the promise not just to
bring lifesaving technologies to developing countries but also to establish
lower price points for products in existing markets -- and, presumably, to
generate a healthy stream of sales in both markets.
The Stanford program hopes to further that idea by combining
its fellows' Indian experience with its Stanford-trained biodesign expertise, to
generate a list of problems that demand better devices. The list is then vetted
to focus on problems that are both urgent and scalable, have the potential for a
big impact, and present good business opportunities.
In the case of the bone drill, the fellows were able to
develop rough, functioning prototypes. But then they hit a wall. They realized
they needed more sophisticated engineering and design help than they could get
in India.

Bone drills enable fluids to be delivered into bone marrow in
less than 60 second--a lifesaver when a patient’s veins have collapsed. These
prototypes by Lunar Design demonstrate the steps from drilling to fluid
delivery. | Photograph courtesy of ABC Family

In India, where a chaotic road system spawns many
accidents and hospitals are often hours away, the need for an inexpensive
alternative vascular access is great.
"The Indians knew they could benefit from having a
world-class group optimize the [bone drill's] engineering and think about the
user interface," Doshi says. "The device needed to be disposable, have a low
part count, be inexpensive, easily assembled, capable of being used by a poorly
trained person, and able to be manufactured cheaply in India."
Doshi pointed the group to Lunar Design, a San Francisco
industrial-design firm. "I told the Lunar team, 'Here are the requirements. I
know you're at full capacity, but this is important. And here's a timeline that
isn't achievable."
This wasn't the first time the designers at Lunar had worked
with Doshi, who is also the founder and chief scientific officer of device
manufacturer Ventus Medical. Lunar had tackled a similar issue with a low-cost
sleep-apnea device it had previously codeveloped for Ventus. For that product
(which won an IDEA gold award in 2010), instead of developing a ne plus ultra
gadget, the team focused on a lower-cost solution that would work for most
potential users, but not all of them. It's what the industry calls the "best
value" solution, not the "best possible" one.
That's a sensible -- but radical -- notion. "The approach in
the U.S. is to come in with big guns and solve a problem with a device that
always works for every person, in every situation, even if it costs more," says
Lunar president John Edson. "That is the model that has been rewarded by
payers."
But as GE's experience illustrates, that is no longer the
sole model -- not just because of the potential of emerging markets but also
because of the skyrocketing cost of health care everywhere.
So Lunar engineered its bone drill to work manually rather
than on expensive batteries. It stripped out nonessential features and made sure
the drill could be operated with only two hands.
The collaboration benefited both sides. The Indians got
access to Silicon Valley's sophisticated engineering and device-development
ecosystem, and the California team got a ramped-up design process, thanks to the
relative ease of testing in India.
"There's a lot more red tape in the U.S.," says Lunar's Matt
Durack. "This device can only prove out on human bones, and we were able to take
months out of the process because of faster access to cadavers in India."
The teams collaborated over Skype and shipped prototypes back
and forth via FedEx. Eventually, they created a device that would sell for
around $20, with no drop in efficacy.
There are still many hurdles before the device will be
available commercially. For one thing, designers have yet to crack the code on
making sure the drill is not reusable, a big issue in India where blood-borne
diseases are often spread with reusable devices. And even if the device clears
regulatory hurdles in India, it will be a while before it could pass the FDA
here and circumvent lobbyists' resistance to cheaper devices.
But the collaborative-development model -- taking advantage
of the best opportunities in each culture -- has the potential to up-end device
development and boomerang back with benefits for health care on both sides. And
the SIB program is training the trainers of the future. "The power of precedent
is extraordinary," says Doshi. "If we're successful, we'll have created not just
a device but a model that others can replicate. This team understood the need
and created something socially impactful. It's what designers love to do."
(A version of this article appears in the July/August 2011
issue of Fast Company.)
|
