|
Biotech Strategy To Develop Simple, Rapid, Indigenous, Low
Cost Medical Devices & Implants
As part of the National Biotechnology Development Strategy-2014 (Biotech
Strategy II), the Department of Biotechnology (DBT) aims to develop simple,
rapid, indigenous, low cost medical devices and implants by applying four major
components i.e. affordability, accessibility, availability and appropriateness.
In fullfilment of the Biotechnology Vision-2020 and realising that biotechnology
has the potential to be a globally transformative intellectual enterprise of
humankind, the DBT had recently issued the draft Strategy to establish India as
a world class biomanufacturing hub for developing and developed markets.
The Strategy aims to produce a large number of medical technology innovators and
also aims to expand multi-disciplinary, team-based program across the country to
train engineers and physicians for clinical immersion through biodesign process
in India. The Strategy aims for standardisation and protocol testing of
biodesign products and also proposes to establish collaboration with various
international institutes and universities.
As part of the Strategy, the DBT will initiate inter-institutional Ph.D
programme, innovation award, overseas fellowships, etc. and will create quality
manpower in engineering school in partnership with medical schools for
multidisciplinary research, skilled technicians, manufacturing engineers and
regulatory staffs.
The Strategy for the next five years includes to establish biodesign
inter-institutional centre at Translational Health Science and Technology
Institute (THSTI), Faridabad with large number of faculty, proper infrastructure
and facilities such as platform technology, validation unit, pre-clinical,
clinical trials; and expansion of biodesign concept in other IITs, medical
schools and institutes across the country. Besides, the DBT will create an
effective National Biodesign Alliance with the partnering institutions (virtual)
with a secretariat at THSTI, Faridabad.
To achieve this Vision-2020, the Biotech Strategy proposes to create replica of
biodesign concept at other IITs, medical schools and institutions; and to
promote ideas generation for medical devices innovation and inter-disciplinary
research. It also proposes lowering the import of medical devices by indigenous
innovation and fast market implementation.
As part of the Strategy, the DBT will introduce healthcare technologies
including biodesign in the curriculum of medical and engineering schools for
undergraduate and post graduate programmes. It will develop infrastructure for
product development such as laboratories for animal studies, prototypes
development and validation studies of the developed products; and manufacturing
capabilities such as cluster capability, low volume incentives (tax) and quality
certification.
(Ref:
http://pharmabiz.com/ArticleDetails.aspx?aid=81590&sid=1)
Medtech Start-up Develops Germ-killing Catheter
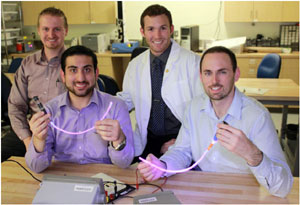
University of Utah bioengineering student Nate Rhodes has come up with a
solution to catheters causing clotting and infection. Along with other students,
he has developed a type of catheter that emits visible light killing bacteria to
prevent infections from occurring.
Rhodes’ team of bioengineering and medical students recently won first place and
$75,000 at the International Business Model Competition hosted by Brigham Young
University. The competition drew more than 2,500 teams from 200 schools
representing 20 countries from around the world.
The students created a start-up company, Veritas Medical, to develop the Light
Line Catheter, using high-intensity narrow spectrum light, which is known to
kill bacteria without any harmful effects to human cells. They have already
filed a utility patent on their technology and will complete laboratory testing
later this year followed by clinical trials beginning next year.
“This competition was a huge validation for what we have created,” said Rhodes,
who gained a master’s in bioengineering this spring. “We have come a long way
since starting this project three years ago, and we hope to finish clinical
trials by 2015 and begin selling our product by 2016.”
Other members of the team include James Allen, a bioengineering graduate; Mitch
Barneck, a bioengineering graduate currently in medical school at Oregon Health
and Science University; Martin de La Presa, a medical student; and Ahrash
Poursaid, who received a bachelor’s degree in bioengineering this spring.
Veritas Medical plans to use the $75,000 in winnings to support further product
development and validation. It has already conducted successful laboratory tests
of its product, and is working towards getting clearance from the US Food and
Drug Administration.
The students previously won more than $20,000 in cash prizes and grants through
other student competitions at the university, including Bench to Bedside, a
medical device competition, the Utah Entrepreneur Challenge, a statewide
business plan competition, and the Entrepreneur Club milestone funding program.
They also received $1,000 from the Baylor New Venture Competition in Waco,
Texas.
“I’m always incredibly impressed by what our students are able to do,” said John
Langell, MD, PhD, MBA, who mentored the team and is the director of the
university’s centre for medical innovation. “We started focusing on training
future health care innovators through our interdisciplinary innovation programs
only a few years ago, and we are already seeing many successful technologies
like this ready to transform the market.”
(Ref:
http://www.medicalplasticsnews.com/industry-news/north-america/medtech-start-up-develops-germ-killing-catheter/
)
|
