|
A safe and adequate blood
supply is essential to the practice of modern medicine. New advances,
particularly in the area of automation, have enhanced both safety and blood
availability and it is the purpose of this study to detail these advances and
their impact on the blood industry.
Sales for the U.S. primary
blood market, which includes the blood and blood components segment and the
plasma derived segment, is currently estimated at $6.6 billion and is expected
to approach $9.9 billion in 2010, rising at an average annual growth rate (AAGR)
of 8.5%. A secondary market includes the products used for collecting,
processing and transfusion. This segment will grow from over $1.8 billion to
nearly $2.5 billion at an AAGR of 7% in the same time period.
Major findings:
-
The driving forces impacting on
this industry are the cost of collection and processing, technological advances,
an aging population and the changes in the incidence of diseases and surgical
procedures and catastrophes requiring blood transfusions.
-
Safety of the blood supply is
the primary issue. Testing of donations has been enhanced with the greater use
of nuclear amplifications testing (NAT) methods.
-
The emergence of recombinant
coagulation factors has allowed for safer clotting factor products. Second and
third generation recombinant products have entered the market.
-
The use of automated
instruments is growing and is now the primary method for blood and plasma
collection. The ability to collect two units of rbcs (red blood cells) from a
single donor is speeding up the donation process. Blood typing and disease
screening has also benefited from automated instruments.
-
An estimated 98% of the units
of rbcs transfused have undergone leukoreduction.
-
Vertical integration, mergers
and acquisitions have resulted in a consolidation of the plasma sector resulting
in only four major players.
-
The industry is unique
comprised of a mixture of FDA regulated for-profit and non-profit organizations.
-
The American Red Cross remains
the leading organization supplying blood although there is competition from
community blood banks.
-
Industry participants include
Abbott, Baxter, Grifols Biologicals, Haemonetics, Immucor, Medimmune, and ZLB
Behring.
-
Forty of 58 (68.9%) recent
patents granted in the areas of blood, blood derivatives and transfusion and
nucleic acid amplification were for basic nucleic acid technology and nucleic
acid devices and related methods.
U.S. Primary and Secondary
Blood Market, through 2010
($ Millions)
| |
2005 |
2010 |
AAGR%
2005-2010 |
|
Plasma Derived Products |
3,404.10 |
4,447.00 |
5.5 |
|
Blood and Blood Components |
3,192.16 |
5,451.65 |
11.3 |
|
Total Primary Blood Market |
6,596.26 |
9,898.65 |
8.5 |
|
Total Secondary Market* |
1,814.40 |
2,544.10 |
7.0 |
* Collection and processing
products including blood donor screening, blood typing equipment and supplies,
collection & transfusion products, processing equipment, supplies and
disposables.
Source: BCC, Inc.
U.S. Primary and Secondary
Blood Market, 2005 and 2010
($ Millions)
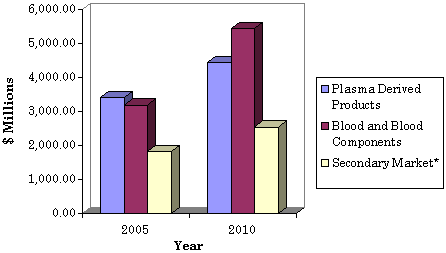
* Collection and processing
products including blood donor screening, blood typing equipment and supplies,
collection & transfusion products, processing equipment, supplies and
disposables.
Source: BCC, Inc.
(Ref : A soon-to-be-released
updated report RC-071V The Blood Industry from Business Communications Company,
Inc. (www.bccresearch.com ;
Contact: Malika Rajan at (203) 853-4266; ext 309;
publisher@bccresearch.com)
*****
|
|