Fecal
Incontinence Device
 Fecal incontinence is the
inability to control bowel movements, causing stool (feces) to leak unexpectedly
from the rectum. It is the inability to control bowel movements and is a common
problem, especially among older adults. The most common cause of FI is damage to
the muscles around the anus (anal sphincters). Vaginal childbirth can damage the
anal sphincters or their nerves, which is why FI impacts women about twice as
often as men. Fecal incontinence is the
inability to control bowel movements, causing stool (feces) to leak unexpectedly
from the rectum. It is the inability to control bowel movements and is a common
problem, especially among older adults. The most common cause of FI is damage to
the muscles around the anus (anal sphincters). Vaginal childbirth can damage the
anal sphincters or their nerves, which is why FI impacts women about twice as
often as men.
Also called bowel incontinence,
fecal incontinence ranges from an occasional leakage of stool while passing gas
to a complete loss of bowel control.
Common causes of fecal
incontinence include diarrhea, constipation, and muscle or nerve damage. The
muscle or nerve damage may be associated with aging or with giving birth.
Whatever the cause, fecal
incontinence can be embarrassing.
Fecal incontinence is even a
bigger issue than urinary incontinence. Close to 50 percent of people in
long-term care suffer from it. That’s an amazing number. U.S. sales of fecal
incontinence products is predicted to hit $1.9 billion by 2018.
The U.S. Food and Drug
Administration has recently allowed marketing of a System for the treatment of
fecal incontinence (FI) in adult women.
“Current treatment options for
fecal incontinence include drugs, dietary changes, exercise, and surgery,” said
William Maisel, M.D., M.P.H., deputy director for science and chief scientist in
the FDA’s Center for Devices and Radiological Health. “The Eclipse System
provides an additional treatment option for women who suffer from this
condition.”
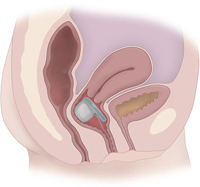
The System is intended to treat
FI in women 18 to 75 years old who have had four or more FI episodes in a
two-week period. The device includes an inflatable balloon, which is placed in
the vagina. Upon inflation, the balloon exerts pressure through the vaginal wall
onto the rectal area, thereby reducing the number of FI episodes. The device is
initially fitted and inflated by a clinician (with the use of a pump) and after
proper fitting, the patient can inflate and deflate the device at home as
needed. The device should be removed periodically for cleaning.
Adverse events associated with
the device included pelvic cramping and discomfort; pelvic pain; vaginal
abrasion, redness, or discharge; and urinary incontinence. All device-related
adverse events were mild or moderate, and none required any significant
intervention (i.e., no surgeries were needed).
(Ref:
http://www.mayoclinic.org/diseases-conditions/fecalincontinence/basics/definition/con-20034575
http://www.postbulletin.com/news/local/medical-tech-companyto-build-plant-in-stewartville/article_d8fb03f8-5729-58e5-b075-645d443c88f8.html
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/
ucm434130.htm)
|

