|
Niti Aayog proposes separate regulator for
medical devices
The ministry had issued a draft notification saying that all
medical devices would come under the category of drugs from December 1 and would
be regulated under the Drugs & Cosmetics Act.
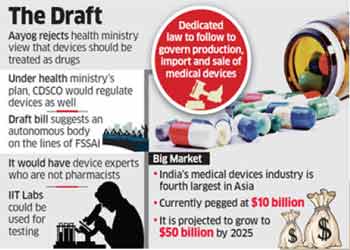
NEW DELHI: Government think tank Niti Aayog has rejected the
health ministry’s proposal to bring medical devices under the Central Drugs
Standard Control Organisation (CDSCO), saying the body does not have the
required expertise. People aware of the matter said the Aayog has instead moved
a draft Bill proposing that medical devices be governed by a separate regulator.
Earlier this month, the ministry had issued a draft
notification saying that all medical devices would come under the category of
drugs from December 1 and would be regulated under the Drugs & Cosmetics Act.
A senior official confirmed to ET that there is some support
for Niti Aayog’s view in the government that it is inappropriate to allow CDSCO
to regulate medical devices as they have expertise in pharmacy/chemicals and not
in devices.
People familiar with the development told ET that the Aayog
has floated a draft Bill to regulate over 6,000 bio medical devices in the
country.
“The Bill proposes a separate regulator for medical devices
on the lines of the Food Safety and Standards Authority of India (FSSAI), an
autonomous body under the health ministry,” one of the persons cited earlier
said, requesting not to be named.
In India, only 23 categories of medical devices are regulated
under the Drugs and Cosmetics (D&C) Act. The ministry’s notification said all
medical devices will be brought under regulation in a phased manner.
It has proposed seven categories of devices intended for use
in human beings or animals as drugs with effect from December 1, 2019, while
ultrasound equipment would be treated as drugs from November 1, 2020.
“Ministry of health should clearly define that its current
regulations to define devices as drugs and their regulation by CDSCO is a
temporary measure till a separate medical devices law and a competent regulatory
authority is formed as devices are not drugs,” Rajiv Nath, forum coordinator of
the Association of Indian Medical Devices Industry said.
India’s medical devices market is the fourth largest in Asia
- after Japan, China and South Korea–at over $10 billion and is projected to
grow to $50 billion by 2025.
https://economictimes.indiatimes.com/industry/healthcare/biotech/healthcare/niti-aayog-proposes-separate-regulatorfor-medical-devices/articleshow/71798635.cms
India Wooing Japanese Medical Device Firms
to Cut Reliance on US
The government is exploring all possibilities to attract
Japanese investment in India’s medical devices sector, a move which is likely to
reduce the country’s dependence on US manufacturers.
Price curbs imposed on medical devices have irked US
companies and become a key trade issue between Washington and New Delhi. Even as
the US is sparring with India over this, the focus is now on fast-tracking
investments from other countries, especially Japan, said people who are part of
the discuss.
PD Vaghela, the secretary at the Department of
Pharmaceuticals under the Ministry of Chemicals and Fertilisers, held a
high-level meeting with industry executives and government officials to discuss
the current status of investments in medical devices, how to maximise Japanese
investments and the regulatory issues. Representatives of Japanese companies
like Omron Healthcare, Teremo Corp, Shimadzu Corp, Sysmex Corp, Horiba India,
HitachiNSE -1.18 % India, Toshiba, CuraNSE 0.00 % Healthcare and the Japan
Federation of Medical Devices Association, and the first secretary at the
Embassy of Japan in India attended the November 8 meeting.
Officials from the Ministry of Health, Department of
Promotion of Industry and Internal Trade, Drug Controller General of India, and
representatives from the CII, Ficci, Andhra Pradesh Medtech Zone and the
Association of Indian Medical Device Industry (AiMed) were also present.
“Top government and industry representatives from Japanese
companies and India deliberated various ways of strengthening the partnership
between both countries,” said a person.
While Japanese brands have been household names in India for
cameras, TVs and automobiles, the US remains the largest exporter of medical
devices to India, followed by Germany, China, Singapore and the Netherlands.
The government is now looking at enhanced cooperation from
Japanese investors to strengthen the domestic capacity of medical devices.
“Economic relations between India and Japan have vast
potential for growth, given the similarities that exist between the two
countries. Vision 2025 outlined by both countries aims to strengthen Indo-Japan
relations by synergising business partnerships, facilitating investments and
creating an empowering environment in India for Japanese investments. The
meeting was held in view of that,” said a senior government official.
India’s medical device market is projected to grow to $50
billion by 2025 from $10 billion now. It is the fourth largest in Asia, after
Japan, China and South Korea. Currently, India has 750-800 medical device
manufacturers, with an average investment of Rs 170-200 million and an average
turnover of Rs 450-500 million. While India allows 100% foreign direct
investment in medical devices on the automatic route, investors have been wary
due to the current regulatory environment.
India’s decision to slash prices of coronary stents by 85% in
2017 and then capping the prices of knee implants affected US exports, US trade
representative had said in 2018.
www.economictimes.indiatimes.com/articleshow/72016205.cms?utm_
source=contentofinterest&utm_medium=text&utm_campaign=cppst
|
